- Research
- Open access
- Published:
Assessment of diaphragmatic role in dyspneic patients with pleural effusion
The Egyptian Journal of Bronchology volume 16, Article number: 69 (2022)
Abstract
Background
Dyspnea that is caused by pleural effusion and affects patients’ quality of life may not be resolved after lung expansion following pleural tapping. This study aims to investigate the role that changes in the diaphragmatic shape and movement play in the development of dyspnea in those patients.
Methods
Thirty patients with pleural effusions and dyspnea were evaluated before and at 24 h after therapeutic thoracentesis. The primary outcomes are to investigate changes in diaphragmatic shape and movement before and after thoracentesis by chest ultrasound to evaluate factors causing effusion-related dyspnoea, while the secondary outcomes are firstly to investigate the effect of pleural effusion on the cardiorespiratory, functional, and diaphragmatic variables in causing dyspnea and secondly to detect the percentages and demographics of patients who experience post-drainage dyspnea improvement.
Results
Thirty patients with age >18 years old who had moderate to massive pleural effusion (either of benign or malignant etiology), with breathlessness were recruited from the Chest Department, Ultrasonography Unit, Ain-Shams University Hospitals. Almost all (96.7%) of the studied patients experienced shortness of breath with a mean modified Borg Score of 5.13 ± 1.78, tapping of varying amounts of pleural fluid ranging from 1000 to 2000 ml.
There is a highly significant improvement in the diaphragmatic excursion, with non-significant improvement in diaphragmatic shape, although nonsignificant correlation between diaphragmatic excursion and functional parameters (M. Borg score, spirometry, and 6MWD: 6-min walk distance), but a significant negative correlation between diaphragmatic excursion and amount of drained effusion (P value 0.045 and 95% CI for OR 1.041–36.779). 63.3% of patients experienced dyspnea improvement after thoracocentesis, and they showed highly significant improvements in M. Borg dyspnea score, spirometry, pulse rate, respiratory rate, 6MWD, and blood oxygen saturation.
Conclusion
In this study, we conclude that the improvement of the diaphragmatic excursion was negatively correlated with the amount of drained effusion, but no significant correlation was detected with the functional parameters and effusion-related indices that may be caused by the small sample size of the study.
Background
Dyspnea is a subjective suffering of breathing difficulty. It affects up to 80% of patients with malignant pleural effusion [1,2,3] and a greater percentage of those with heart failure-associated effusions [4]. It was found that pleural effusions can have a large effect, on breathing, quality of life [5], sleep [6], and exercise capacity [7].
It was previously thought that the presence of dyspnea usually depends on the effusion amount, but other factors like the patient’s cardiopulmonary condition and anemia are playing pivotal roles [8]. Although post-drainage lung expansion results in breathlessness improvement, changes in the diaphragmatic shape and movement recently are found to have the main role in dyspnea development in those patients [8, 9].
This work studies the changes in diaphragmatic shape and movement following thoracentesis and evaluation of other factors contributing to effusion-related dyspnea for better management and decision-making for those patients.
Methods
This was a prospective cohort study conducted at the Ultrasonography Unit and Pulmonary Function Unit, Chest Department, Ain Shams University Hospitals, in Cairo, Egypt. Using Epi Info 7 program for the sample size calculation, with a margin of error = 10% and at 95% confidence level, the sample size of at least 30 participants was needed.
The primary outcomes are to investigate changes in diaphragmatic shape and movement before and after thoracentesis by chest ultrasound to evaluate factors causing effusion-related dyspnea, while the secondary outcomes are firstly to investigate the effect of pleural effusion on the cardiorespiratory, functional, and diaphragmatic variables in causing pre-drainage dyspnea and secondly to detect percentage and demographics of patients who experience post-drainage dyspnea improvement.
Inclusion criteria
Thirty patients with inclusion criteria are as follows: age >18 years old with pleural effusion (either of benign or malignant etiology) ≥ 25% of hemithorax and had breathlessness. Convenient non-random sampling was used to recruit the study sample. All patients fulfilling the inclusion criteria were included in the study till the completion of the sample size.
Exclusion criteria
The exclusion criteria are as follows: any loculated, complicated pleural effusion that necessitates surgical interventions, pleural effusion in children, mechanically ventilated patients with pleural effusion, bedridden or immobile patients with pleural effusion, patients with mental illness, pleural effusion associated with trapped lung (e.g., presence of endobronchial lesion), pleural effusion in patients with chronic liver failure, hypoalbuminemia, and any associated cause of rapid transudation.
After informed written consent from all enrolled patients or their legal guardians, all were subjected to a thorough history taking and clinical examination, standard full-size posteroanterior CXR at the time of pleural effusion diagnosis, baseline pre-drainage evaluations which included vital signs, oxygen saturation by pulse oximetry, breathlessness score (resting Borg score), serum albumin, pleural pH, transthoracic ultrasound to evaluate shape and movement of the diaphragm, spirometry, 6-min walk test (distance walked in 6 min on a straight walking track), measured according to the American Thoracic Society guidelines [10]. All of these pre-parameters were repeated again after 24–36 h from thoracentesis of at least 1000 cc pleural fluid.
Spirometry
It was performed according to the standard practice discussed in detail previously [10] using a Viasys Health Care spirometer, D-97204 Hochberg, Germany. The spirometry indices recorded were forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio.
Six minute walk test (6MWT)
A 6-min walk test (6MWT) was done following the American Thoracic Society’s guidelines for the 6MWT. The patients were asked to walk for 6-min over a 30-m hallway at their own pace. The same examiner administered each examination without any encouragement. The test was stopped if patients suffered chest pain, extreme dyspnea, muscle cramps, disorientation, malaise, or hypotension. At end of the test, the total distance walked was recorded in meters. A pulse oximeter was used to measure peripheral oxygen saturation (Spo2) and heart rate before and after the 6MWT (model LOX100C; Lepu Medical, Inc; Heartcare, China) [10].
Ultrasonographic evaluation
Diaphragm ultrasound was done using MindrayDP-1100Plus (2015) Shanghai, China, ultrasonographic machine for the assessment of diaphragmatic mobility and diaphragmatic excursion at the side of effusion.
Patient position
The patient would be in a supine position, and the researcher sat down on a chair on the right side of the bed at the level of the patient’s abdomen; the ultrasound device was beside him at the level of the head of the patient and then the examination was done to the affected side of effusion. The advantages of the supine position are as follows: less overall variability, less side-to-side variability, greater reproducibility, and excursion were known to be greater in the supine position for the same volume inspired than in sitting or standing positions [11].
Diaphragmatic excursion
The examination was done using a 3.5C (bandwidth 2–5 MHz) convex-phased array probe (low-frequency probe with greater depth and allowing to assess excursion), with B mode set as the default mode on the device screen. The probe of the ultrasound was put at an anterior axillary line, right/left subcostal after application of the ultrasound gel and is directed medially, cephalic, and dorsally using the liver/spleen as an acoustic window for better illustration of the diaphragm. First, switch to M mode observing the diaphragmatic movement during inspiration and expiration, then the freeze button on the ultrasound device is pressed, followed by measurement of the difference between the diaphragmatic position during inspiration and expiration, and lastly, the diaphragmatic excursion during breathing was recorded.
Thoracentesis procedure
Thoracentesis guided with chest ultrasound privileged HCP according to hospital policies and procedures was performed by the authors who are a trained pulmonologist using the standard technique in pleural tapping. The procedure was terminated if the spontaneous cessation of pleural fluid drainage or if the patient experienced discomfort with increasing symptoms such as coughing, dyspnea, chest pain, and/or vasovagal manifestations [12].
All the study steps were consistent with the ethical principles of the Declaration of Helsinki for medical research involving human subjects and were approved by the Faculty of Medicine, Ain Shams University, FMASU R 08/2020/2021.
Statistical data analysis
It was done in line with the objectives by using the SPSS program (Statistical Package for Social Sciences) software version 18. Qualitative variables were presented as percentages and Quantitative variables were presented as mean ± SD. Student’s “t” test and Pearson’s correlation coefficient were used as a test of significance, p<0.05 was considered as significant.
Results
This was a prospective cohort study that included 30 patients who had pleural effusion, and 53.3% of them were males. Their mean ages were 53.70 ± 15.57 ranging from 18 to 72 years, and their mean BMI was 27.89 ± 6.96 ranging from 13.84 to 40 kg/cm2. The majority of them had secondary education (43.3%), and 86.7% were married. One fifth of them had no comorbidities. Eighty percent of them had different comorbidities and associated causative conditions such as diabetes mellitus, hypertension, chronic kidney disease, tuberculosis, breast cancer, pneumonia, adenocarcinoma lung, and anemia representing (13.3%, 16.7%, 6.7%, 6.7%, 16.7%, 10%, 6.7%), respectively (Table 1).
Almost all (96.7%) of the studied patients experienced shortness of breath Their mean modified Borg Score was 5.13 ± 1.78 ranging from 2 to 9. Less commonly chest pain (70%), followed by dry cough and fever (13.3%, 6.7%), respectively (Table 2).
Chemistry of almost all (96.7%) of the involved pleural effusions was exudative, and 56.7% of them were left-sided which was mainly moderate to massive (from three to five as CXR classification done). More than 70% of the effusion causes were due to malignancy with adenocarcinoma, metastatic adenocarcinoma, mesothelioma, and NHL (40%, 23.3%, 6.7%, and 3.3%), respectively. 23.3% of the effusions were related to infections. The only cause of transudate pleural (3.3%) effusion was heart failure. Tapping of different varying amounts of pleural fluid ranging from 1000 to 2000 ml and stoppage of tapping was done due to chest pain and coughing mainly which occurred in 36.7% and 30%, respectively (Table 3).
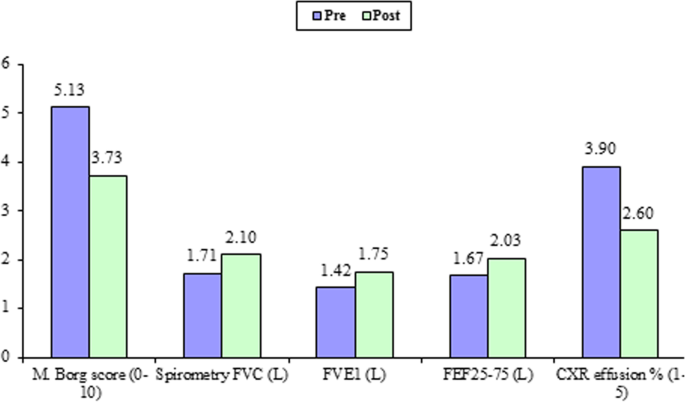
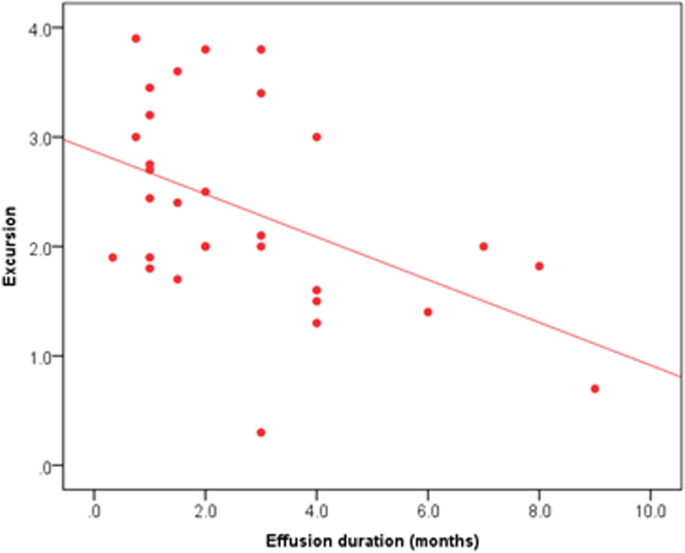
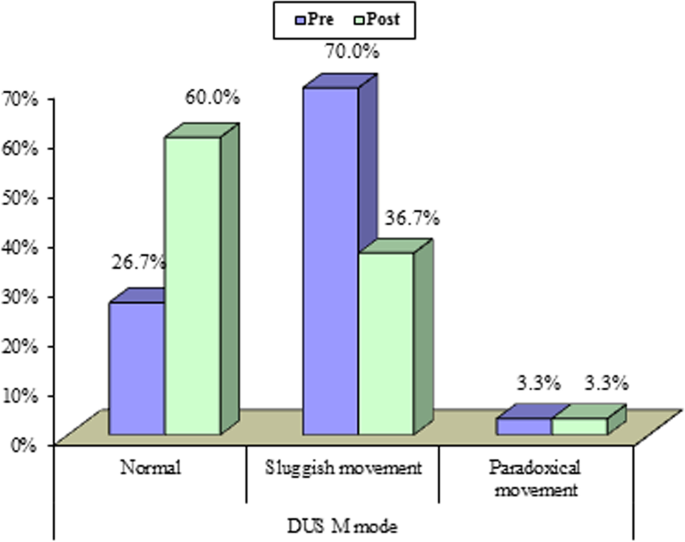
All 30 participants completed all the pre- and post-thoracentesis tests, they showed highly significant improvements in M. Borg dyspnea score, FVC (L), FVC (%), FEV1 (L), FEV1(%), pulse rate (beat/m), respiratory rate, diaphragmatic excursion, and 6-min walk distance (6MWD) with (P value 0.001) for all except blood oxygen saturation (P value 0.003) (Fig. 1). Also, it was detected that diaphragmatic excursion was affected significantly by effusion duration (Fig. 2). These highly significant improvements were in the form of a highly significant increase in all previously mentioned pre thoracentesis functional assessments except pulse rate and respiratory rate (they were highly significantly decreased). They also showed a significant change in the diaphragmatic movement by M mode chest ultrasound (P value 0.03) (Fig. 3). No significant change was shown in the diaphragmatic shape by B mode chest ultrasound (P value 0.781) after thoracentesis (Table 4).
No significant correlation was found between the improvement of diaphragmatic excursion and neither the studied functional assessments (spirometry, 6 MWD) nor effusion-related indices, but the amount of effusion drained was found to be negatively correlated with the improvement of diaphragmatic excursion (P value 0.043) (Table 5).
Discussion
Breathing difficulty is an annoying symptom that affects the quality of life in patients with pleural effusions, and it is composed of a number of perceptions, including the sensation of effort, chest tightness, and air hunger [3]. It was found that pleural effusions can have a large effect on breathing, quality of life [5], sleep [6], and on exercise capacity [7].
It was previously thought that the presence of dyspnea usually depends on the effusion amount, the patient’s cardiopulmonary condition, and the presence of associated anemia. Many confounding and complicated factors contribute to effusion-related dyspnoea which remained not so clear. Better management and decision-making for those patients need more studies hoping to understand the underlying challenging process well. This work studied the change in diaphragmatic function following thoracentesis and evaluated it as one of the factors contributing to effusion-related dyspnoea.
Almost all the study participants (96.6%) were complaining of shortness of breath with their M. Borg dyspnea score was 5.13 ± 1.78 ranging from 2 to 7. After aspiration of 1263.33 ± 341.88 ml ranging from 1000 to 2000 ml of pleural fluid, a highly significant decrease in M. Borg dyspnea score was documented 3.73 ± 1.20 ranging from 1 to 7 with a P value of 0.001. Pleural effusions are associated with abnormalities in gas exchange, respiratory mechanics, respiratory muscle function, and hemodynamics, but the association between these abnormalities and breathlessness remains unclear. Also, the response following thoracentesis remains uncertain [13].
In this work, improvement of patients’ dyspnea score after thoracentesis was accompanied by a highly significant improvement in all documented spirometric indices FVC (L), FVC (%), FEV1 (L), and FEV1(%) (1.71 ± 0.49 versus 2.10 ± 0.50, 45.28 ± 10.93 versus 55.37 ± 10.90, 1.42 ± 0.45 versus 1.75 ± 0.46, and 45.89 ± 12.19 versus 56.97 ± 13.42, respectively) with a P value of 0.001.
Some studies have demonstrated significant improvement after the removal of as little as 800 mL of pleural fluid [14]. Uniform and slow withdrawal of the pleural fluid is essential. The incidence of re-expansion pulmonary edema is increased by the rapid removal of large volumes of fluid. Finally, the inversion of a hemidiaphragm documented by chest sonography may explain the discrepancy between previous studies [15, 16].
Others demonstrated that the PM (paradoxical movement) group showed significant improvements in lung function, gas exchange, and dyspnea following thoracocentesis, whereas the NPM (non-paradoxical movement) group did not show any significant change in any parameter. The NPM group also had better lung function, gas exchange, and dyspnea before thoracocentesis [17].
On a deep look for these improvements, we found that there were no significant correlations between any of these functional indices and the difference in the diaphragmatic excursion (excursion improvement after thoracentesis) except in the amount of effusion which was found to be negatively correlated with the improvement in the diaphragmatic excursion. Also, no effusion-related factors (type, duration, cause, side) were found to be correlated to the improvement in a diaphragmatic excursion. Different attributions could explain these non-significant correlations; in the small sample size of this study population, the etiology of pleural effusion as 73.3% of patients had malignant pleural effusion that could impair diaphragmatic excursion by more than benign causes of pleural effusion. Also, other factors such as the rate of pleural fluid accumulation (almost all effusions were exudates) and the amount of thoracic involvement of effusions were mainly from 25 to 50%, only one case showed a paradoxically moving diaphragm. Sometimes when the amount of tapping exceeds 1L, rapid lung inflation and re-expansion pulmonary edema with a larger amount of tapped effusion are associated with an irritative cough that affects the voluntary diaphragmatic function and hence its excursion.
Different studies agree that the increase in lung volumes is small and does not correlate [18] or poorly correlate [14] with the amount of aspirated pleural fluid irrespective of whether the effusion is a transudate or exudate [19]. Animal and human studies suggest that expansion in the thoracic cage is the principal mechanism by which extra volume is generated to accommodate the effusion and helps preserve lung volumes. In anesthetized dogs, infusion of saline inside the pleural cavity increased thoracic cage volume by two thirds of the total volume instilled but only reduced the functional residual capacity (FRC) by one third of the total volume instilled [20, 21].
The increase in thoracic cage volume was achieved mainly through the downward displacement of the diaphragm [21, 22]. In rats, bilateral pleural effusions also increased both the anteroposterior and lateral rib cage diameters [23]. Acute pleural effusions increase respiratory system elastance by increasing lung elastance, likely via lung distortion and decreases in FRC. The effect of pleural effusions on lung resistance is unclear. Pleural effusions do not appear to alter chest wall elastance or resistance [21, 23]. Our knowledge of the effect of effusion on lung volume in humans comes mainly from changes measured pre-thoracentesis and post-thoracentesis. Although thoracentesis can improve the forced expiratory volume in the first second (FEV1) vital capacity [17, 23], the magnitudes of increase are highly variable and often do not correlate with the volume of fluid drained. The effect of thoracentesis on FEV1 and vital capacity appears to be greater in patients with the paradoxical movement of their hemidiaphragm [23]
This study had some limitations; in the small sample of the study population, patients with pleural effusions should be selected almost matched at least as regards BMI, etiology, and size of pleural effusions. Adding whole lung volumes not only a spirometer will add accurate assessment in relation to diaphragmatic assessment pre- and post-thoracentesis.
Conclusion
Thoracentesis improves lung functions including spirometry and 6MWD, but not all of these improvements after thoracentesis were correlated to the improvement in a diaphragmatic excursion.
Availability of data and materials
The data sets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- DM:
-
Diabetes mellitus
- HTN:
-
Hypertension
- CKD:
-
Chronic kidney disease
- T.B:
-
Tuberculosis
- M. Borg:
-
Modified Borg
- RR:
-
Respiratory rate
- DUS:
-
Diaphragmatic ultrasound
- MWD:
-
Minute walk distance
- FVC:
-
Forced vital capacity
- FEV1:
-
Forced expiratory volume in the first second
- FEF:
-
Forced expiratory flow
- PM:
-
Paradoxical movement
References
Valdés L, San-José E, Ferreiro L et al (2013) Combining clinical and analytical parameters improves prediction of malignant pleural effusion. Lung 191:633–643
Tanrikulu AC, Abakay A, Kaplan MA et al (2010) A clinical, radiographic and laboratory evaluation of prognostic factors in 363 patients with malignant pleural mesothelioma. Respiration 80:480–487
Nieder C, Kämpe TA, Engljähringer K (2018) Does patient-reported dyspnea reflect thoracic disease characteristics in patients with incurable cancer? Anticancer Res 38:901–904
Nazemiyeh M, Dorraji A, Nouri-Vaskeh M et al (2019) Congestive heart failure is the leading cause of pleural effusion in the north-west of Iran. J Cardiovasc Thorac Res 11:244–247
Sivakumar P, Saigal A, Ahmed L (2020) Quality of life after interventions for malignant pleural effusions: a systematic review. BMJ Support Palliat Care 10:45–54
Marcondes BF, Vargas F, Paschoal FH et al (2012) Sleep in patients with large pleural effusion: impact of thoracentesis. Sleep Breath 16:483–489
Cartaxo AM, Vargas FS, Salge JM et al (2011) Improvements in the 6-min walk test and spirometry following thoracentesis for symptomatic pleural effusions. Chest 139:1424–1429
Muruganandan S, Azzopardi M, Thomas R et al The PLeural effusion and symptom evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion. Eur Respir J. https://doi.org/10.1183/13993003.00980-2019
Porcel JM PLEASE, take a deep breath. Eur Respir J 55:2000501. https://doi.org/10.1183/13993003.00501-2020
Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D et al (2014) An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 44:1428–1446
Eugenio O, Michael C, John P, Kiran J (2001) Ultrasound evaluation of diaphragmatic motion. J Ultrasound Med 20:597–604
Antunes G, Neville E, Duffy J, Ali N, Pleural Diseases Group, Standards of Care Committee, British Thoracic Society (2003) BTS guidelines for the management of malignant pleural effusions. Thorax 58(suppl 2):ii29–ii38
Thomas R, Jenkinsd S, Eastwood PR, Leea G, Singh B (2015) Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 21(4):338–345
Light RW, Stansbury DW, Brown SE (1986) The relationship between pleural pressures and changes in pulmonary function after therapeutic thoracentesis. Am Rev Respir Dis 133:658–661
Pien GW, Gant MJ, Washam CL, Sterman DH (2001) Use of implantable pleural catheter for trapped lung syndrome in patients with malignant pleural effusion. Chest 119:1641–1646
Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P (2000) Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med 162:1534–1538
Wang LM, Cherng JM, Wang JS (2007) Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology 12:719–723
Brown NE, Zamel N, Aberman A (1978) Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest 74:540–542
Light RW, Stansbury DW, Brown SE (1981) Changes in pulmonary function following therapeutic thoracocentesis (abstract). Chest 80:341
Dechman G, Mishima M, Bates JH (1994) Assessment of acute pleural effusion in dogs by computed tomography. J Appl Physiol 76:1993–1998
Krell WS, Rodarte JR (1985) Effects of acute pleural effusion on respiratory system mechanics in dogs. J Appl Physiol 59:1458–1463
Sousa AS, Moll RJ, Pontes CF et al (1995) Mechanical and morphometrical changes in progressive bilateral pneumothorax and pleural effusion in normal rats. Eur Respir J 8:99–104
Dechman G, Sato J, Bates JH (1993) Effect of pleural effusion on respiratory mechanics, and the influence of deep inflation, in dogs. Eur Respir J 6:219–224
Acknowledgements
All deep thanks, gratitude, and appreciation to all participants who accepted to share their data in this study.
Funding
Not available.
Author information
Authors and Affiliations
Contributions
HM has put the design of the work and data acquisition and analysis, interpreted the data, and revised the manuscript. HG shared data interpretation and extensively shared in writing the results and was a major contributor to writing the manuscript. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee Board of Ain Shams University and in accordance with the Declaration of Helsinki (FWA: 000017585) (FMASU R 08/2020/2021). Informed written consent was taken from all enrolled patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shalaby, H.M., Ezzelregal, H.G. Assessment of diaphragmatic role in dyspneic patients with pleural effusion. Egypt J Bronchol 16, 69 (2022). https://doi.org/10.1186/s43168-022-00170-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-022-00170-6